(Catalytic Chemistry)

(Catalytic Chemistry)

(b.1942), B.S.(1964, Waseda), M. Engr.(1966, Waseda), Ph.D.(1969, Waseda),
Res. Assoc.(1969, Waseda), Post Doc.(1971-1973, Alberta), Assoc. Prof.(1975,
Waseda), Prof.(1980, Waseda).
Japan Petroleum Institute Award for Distinguished Papers(1977), The
Fuel Society of Japan Award for Young Scientists(1981), The Award for Catalyst
Preparation Chemistry of the Catalysis Society of Japan (1992), The Catalysis
Society of Japan Award (1996).
keywords
Catalysis / Energy and Fuel Chemistry / Environmental Catalysis / Heterogeneous Catalysis / Membrane Reactor / Hydrogen-Permselective Membrane / Zeolite / Super Conductive Oxide
Tel: +81-3-5286-3203
Fax: +81-3-3200-5349
E-mail:ekikuchi@waseda.jp
Research Interests
Catalysts, as the key material for chemical conversion, are indispensable
for efficient and environmentally benign chemical processes, and also for
abatement of environmentally hazardous substances. The main research interests
are related to high-performance catalysts and catalytic processes for energy
and environmental issues. Some recent findings in our laboratory are illustrated
in the following.
Environmental Catalysis
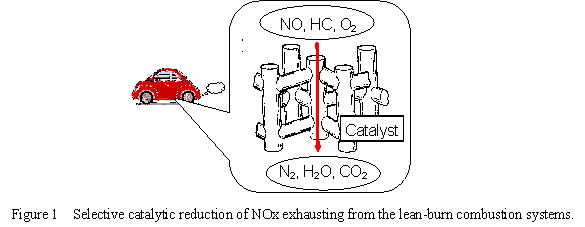
Abatement of harmful compounds in atmosphere, such as nitrogen oxides that cause acid rain or smog and ozone problems, is one of the essential tasks for modern chemistry. Selective reduction of NOx in exhausts from the lean-burn combustion system such as diesel engines was achieved by use of methane as a reductant and Pd catalysts supported on zeolites, and the catalytic activity was found to be stabilized by co-existence of Co cations.
Hydrogen energy
Hydrogen is an important molecule for chemical industries and its importance as energy in the future is increasing. Production of hydrogen is industrially achieved by chemical reduction of steam (steam reforming) by use of supported nickel catalysts. When nickel is supported on particular composite oxides such as perovskite, it has been shown that the lattice oxygen anions directly participate in chemical reactions. This concept will lead to the new type of high-performance steam reforming catalyst.
Integrated catalytic system (Membrane reactor)
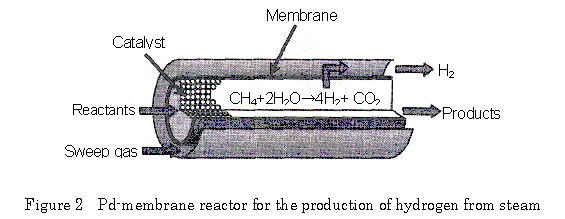
Palladium membrane has an absolute selectivity for hydrogen permeation. When a reaction producing hydrogen is performed in a reactor coupled with separation of hydrogen through the palladium membrane, the reaction is accelerated and the thermodynamic equilibrium can be shifted to accomplish favorable high conversion. Application to steam reforming of methane, which requires elevated temperatures as high as 1100K due to thermodynamic limitation, enables an efficient hydrogen production even around 800K (Fig. 2), as practically demonstrated in the polymer electrolyte fuel system.
Similarly, oxygen-permeable membranes can set up a membrane reactor, in which oxygen in air can be separated and supplied to the oxidation reaction system through the membrane. Mixed composite oxides such as perovskite compounds were successfully transformed to asymmetric membranes. The membrane reactor can be applied to partial oxidation of natural gas to hydrogen and syngas (a mixture of hydrogen and carbon monoxide).
Representative Publications
1. "Steam Reforming of Methanol on Ni/Al2O3 Catalyst in a Pd-membrane Reactor", Journal of the Japan Petroleum Institute, 46, 93-98 (2003)
2. "Co Cation Effects on Activity and Stability of Isolated Pd(II) Cations in Zeolite Matrices for Selective Catalytic Reduction of Nitric Oxide with Methane", Journal of Catalysis, 211, 75-84 (2002)
3. "Effect of NH4+ Exchange on Hydrophobicity and Catalytic Properties of Al free Ti-Si-beta Zeolite", Journal of Catalysis, 199, 41-47 (2001).
4. "Palladium Species in Pd/H-ZSM-5 Zeolite Catalysts for CH4-SCR", Research on Chemical Intermediates, 26, 55-60 (2000).
5. "Determination of Active Palladium Species in ZSM-5 Zeolite for Selective Reduction of Nitric Oxide with Methane", Applied Catalysis B, 23, 247-257 (1999).
6. "Role of Zeolite Structure on Reduction of NOx with Methane over In- and Pd-based Catalysts", Catalysis Today, 45, 139-145 (1998).
7. "Intrapore Catalysis in Reduction of Nitric Oxide with Methane", Catalysis Today, 42, 159-166 (1998).
8. "The Effect of Zeolite Structure on Creation of InO+ Active Sites for NOx Reduction with Methane", Microporous and Mesoporous Materials, 21, 533-540 (1998).
9. Hydrogen-Permselective Membrane Reactors", CaTTech, 1, 67 (1997)
10. "Catalytic Properties and Crystalline Structures of Manganese-Promoted Iron Ultrafine Particles for Liquid Phase Hydrogenation of Carbon Monoxide" Applied Catalysis. A, 96, 125 (1993)
11. "Gallium Ion-exchanged Zeolite as a Selective Catalyst for Reduction of Nitric Oxide with Hydrocarbons under Oxygen-rich Conditions", Catalysis Letters, 17, 303 (1993)
12. "Hydrogen Permeable Palladium-Silver Alloy Membrane Supported on Porous Ceramics", Journal of Membrane Science, 56, 315 (1991)
13. "The Effect of Spilled-over Hydrogen on the Activity of Montmorillonite Pillared by Aluminum Oxide for Conversion of Trimethylbenzenes", Journal of Catalysis, 106, 38 (1987)
14. "Catalytic Synthesis of Hydrocarbons from Carbon Monoxide and Hydrogen on Lamellar Compound of Graphite Intercalated by Ferric Chloride", Journal of Catalysis, 57, 27 (1979)
15. "The Reaction of n-Heptane on Rhodium Catalysts in the Presence of Steam", Journal of Catalysis, 46, 382 (1977)
16. "Studies of the Enhancement of Hydrogen Adsorption during H2-O2 Titration on Supported Pt Catalysts", Journal of Catalysis, 34, 132 (1974)
17. "Hydrogenolysis and Isomerization of n-Pentane on Groupá[ Transition Metals", Journal of Catalysis, 22, 226 (1971)
18. "Hydrogenolysis of n-Pentane on Nickel Catalyst", Journal of Catalysis, 15, 217(1969)
Back to: