(Inorganic Chemistry)

(Inorganic Chemistry)

(b. 1960), B.S. (1983, Waseda), M.Engr. (1985, Waseda), Ph.D (1988,
Waseda), Post Doc (1989-90,Mass. Inst. Tech.), Assistant Prof. (1990, Waseda),
Associate Prof. (1992, Waseda).
Award for Young Clay Scientist (1989), CSJ Award for Advancement in
Ceramic Science and Technology (1992).
Inorganic Materials Chemistry / Ceramic Materials / Preceramic / Sol-Gel Processing / Electro-Conductive Oxides
Room 65-406
3-4-1 Okubo, Shinjuku-ku, Tokyo 169-8555, Japan
Tel: +81-3-5286-3204
Fax: +81-3-5286-3204
E-mail:ys6546@waseda.jp
Research Interests
Our research group is concerned with the development of chemical routes to inorganic materials and inorganic-organic hybrids. Our objectives are to discover new materials and to provide novel synthetic routes to materials exhibiting interesting properties. Our research program makes extensive use of both molecular and solid-state chemistries.
"Chimie douce" approach to novel two-dimensional protonated solids.
A "chimie douce" approach can produce a variety of new inorganic
materials that cannot be prepared by conventional high-temperature solid-state
reactions. We are working on a novel route to the preparation of new protonated
compounds from Aurivillius phases (Bi2An-1BnO3n+3) via selective leaching
of bismuth oxides sheets. New compounds prepared through this route include
protonated forms of ion-exchangeable layered perovskites with new compositions
and a double-octahedral layered tungstic acid.(see a scheme in the previous
page)
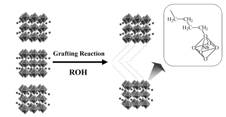
Chemical routes to two-dimensional inorganic-organic hybrids of ion-exchangeable
layered perovskites via intercalation and surface modification.
The accommodation of organic molecules and polymers in the interlayer space
of layered compounds can produce a variety of hybrid materials with ordered
structures. We are developing hybrid materials based on ion-exchangeable
layered perovskites. Intercalation via ion-exchange and acid-base mechanisms
is an established technique for the preparation of these hybrids. We are
also interested in modification of the interlayer surface via grafting
reactions with molecules, such as alcohols, since this approach can create
various interlayer environments. We are currently also studying organic
reactions, such as hydrosilylation, involving organic groups grafted on
the interlayer surface as new methods of interlayer environment modification.
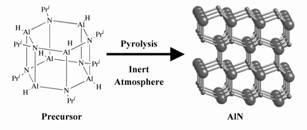
Pyrolytic conversion to non-oxide ceramics from inorganic and organometallic
compounds.
A pyrolytic route from inorganic and organometallic compounds to non-oxide
ceramics can be applicable to film and fiber production, if the precursors
are soluble in organic solvents or fusible. We havedeveloped AlN precursors
possessing oligomeric cage-type structures and have uncovered their conversion
mechanism. We also employ these cage-type molecules for the preparation
of AlN-based ceramic composites through combination with other molecules,
such as cyclic compounds.
Chemical modification of metal alkoxides and the application of modified
metal alkoxides to sol-gel processes for oxides and inorganic-organic hybrid
preparation.
Sol-gel processes have been widely employed in the preparation of various
oxides and inorganic-organic hybrids. Chemical modification is often required
to control the hydrolysis behavior of highly-reactive metal alkoxides.
We are developing methods for modifying of transition metal alkoxides with
catechol to this end. We are also applying an NMR technique to achieve
better understanding of the hydrolysis and condensation processes of metal
alkoxides by monitoring the processes in situ.
Representative Publications
1. "Interlayer Surface Modification of the Protonated Triple-Layered Perovskite HCa2Nb3O10・xH2O with n-Alcohols," Langmuir, 19, 9473-9478 (2003).
2. "A Layered Tungstic Acid H2W2O7・nH2O with a Double-Octahedral Sheet Structure: Conversion Process from an Aurivillius Phase Bi2W2O9 and Structural Characterization," Inorg. Chem., 42, 4479-4484 (2003).
3. "Intercalation Behavior of n-Alkylamines into a Protonated Form of a Layered Perovskite Derived from Aurivillius Phase Bi2SrTa2O9," Chem. Mater., 15, 632-635 (2003).
4. "Reactions of Alkoxyl-Derivatives of a Layered Perovskite with Alcohols: Substitution Reactions on the Interlayer Surface of a Layered Perovskite," Chem. Mater., 15, 636-641 (2003).
5. "Conversion of a Precursor Derived from Cage-Type and Cyclic Molecular Building Blocks into Al-Si-N-C Ceramic Composites," J. Am. Ceram. Soc., 85, 59-64 (2002).
6. "Conversion Process of Strontium-Titanium Bimetallic Methoxyethoxide Precursor into SrTiO3 via Hyrolysis/Calcination," J. Am. Ceram. Soc., 85, 2195-2199 (2002).
7. "Preparation and HREM Characterization of a Protonated Form of a Layered Perovskite Tantalate from an Aurivillius Phase Bi2SrTa2O9 via Acid Treatment," Inorg. Chem., 40, 5768-5771 (2001).
8. "New Conversion Reaction of an Aurivillius Phase into the Protonated Form of the Layered Perovskite by the Selective Leaching of the Bismuth Oxide Sheet," J. Am. Chem. Soc., 121, 11602-11603 (1999).
Back to: